Hepat-omics project - Identification of novel peripheral blood biomarkers for liver disease in β-thalassaemia patients through the use of ‘omics’ technologies.
This project aims primarily to identify potential non-invasive, novel serum diagnostic biomarkers for different stages of liver disease (fibrosis and cirrhosis) in β-thalassaemia patients using combined data from proteomics, metabolomics and transcriptomics. This project is funded by the national Research Promotion Foundation/ Research and Innovation Foundation under the RESTART Programs 2016 – 2020/ Excellence Hubs (EXCELLENCE/0918/118).
Project title: Identification of novel peripheral blood biomarkers for liver disease in β-thalassaemia patients through the use of ‘omics’ technologies.
Project acronym: Hepat-omics
β-thalassaemia is a hereditary haemoglobinopathy caused by reduced or absent expression of β-globin. In its most serious form it leads to severe anaemia and related complications in major organs. Unless treated, it results in childhood death. In the overwhelming majority of patients, treatment is symptomatic rather than curative and involves regular blood transfusions and iron chelation therapy. In Cyprus and other Mediterranean countries β-thalassaemia has traditionally been a major health problem.
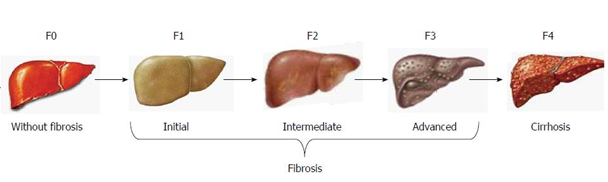
As the management of β-thalassaemia becomes increasingly effective, the disease is progressively shifting away from a lethal childhood anaemia towards a chronic haemoglobinopathy, where the primary causes of mortality are the related disease complications. Until recently, the main cause of death for adult β-thalassaemia patients in mid- to high-income courtiers was cardiac disease, brought about by organ damage due to iron overload. In the last few years a new trend is being observed, with liver disease steadily gaining prominence. As in the case of cardiac disease, iron overload in the liver is one of the main causes of organ damage. Initial liver fibrosis, if unchecked, can progress to cirrhosis and potentially to hepatocellular carcinoma (HCC).
Figure 1. Liver fibrosis progression and staging (Ramos-Lopez, Martinez-Lopez, Roman, Fierro, & Panduro, 2015), doi:10.3748/wjg.v21.i41.11552.
Having a method to easily, reliably and quickly detect and stage liver fibrosis and cirrhosis would greatly aid in guiding more personalised management of iron chelation for β-thalassaemia patients to avoid further liver damage. Additionally, as research into the treatment of liver fibrosis brings us ever closer to effective therapeutic options for this condition, accurate staging of liver disease becomes even more relevant.
Although there is evidence that it is not an infallible test, liver biopsy is the gold standard for the accurate evaluation of liver fibrosis and cirrhosis. This is a rather invasive method with inherent dangers, which is increasingly resisted by β-thalassaemia patients. Given its invasive nature it is not a routine examination technique but is reserved for the most serious cases. Although non-invasive imaging techniques are available, these are not as accurate as the biopsy and suffer from reduced accuracy, particularly for distinguishing between the different early stages of liver fibrosis. Existing serum biomarkers present with their own accuracy problems. In addition, the vast majority of these biomarkers (and imaging techniques) are identified, developed and validated using patients where the aetiology of liver disease was not iron overload. Therefore, the accuracy of these biomarkers in thalassaemia patients may be even lower than for other patients with liver disease.
This project aims primarily to identify potential non-invasive, novel serum diagnostic biomarkers for different stages of liver disease (fibrosis and cirrhosis) in β-thalassaemia patients using combined data from proteomics, metabolomics and transcriptomics. Additionally, we will use these techniques to run some initial validation tests for some of the molecules we identify, as well test the accuracy of some of the more recently identified serum biomarkers in our thalassaemic patient population with iron overload-related liver disease. Lastly, the establishment of well-characterised patient and sample cohorts and a sample biobank will provide us with the opportunity to investigate in future longitudinal studies the possible prognostic value of any biomarkers we identify.
The specific aims of the study are:
- Establishment of a well characterised patient and control cohort for the study
- Performance of proteomics, metabolomics and transcriptomics analysis using plasma samples from β-thalassaemia patients and healthy controls
- Bioinformatics analysis of ‘omics’ data and the integration of the data from all three ‘omics’ platforms
- Investigation of the diagnostic value of the identified serum biomarkers in the β-thalassaemia cohort
The proposed project is a basic research project in the field of health that will generate advanced knowledge through the integration of cutting edge ‘omics’ techniques. This new information has the potential to lead to the development of novel, non-invasive serum biomarkers that will enable early detection of liver fibrosis in β-thalassaemia patients, and help prevent the development of serious liver disease which is rapidly becoming a major cause of mortality in this patient group. Such an outcome would have a tremendous impact on patient quality of life - especially for older individuals who are more likely to suffer from liver disease - enhancing their productivity and the utilisation of the healthcare resources of the country.
The research will be performed primarily at the Cyprus Institute of Neurology and Genetics (CING), which is one of Cyprus’ leading biological research centres in terms of both facilities and personnel. The Molecular Genetics Thalassaemia Department, which will be leading the project, has been performing research on different aspects of the β-thalassaemia for the last 20 years with numerous publications and grants awarded to it. The ‘omics’ experiments will be carried out on site at the CING mass spectrometry and Next Generation Sequencing facilities and the analyses will be performed by the CING Bioinformatics group, which although relatively recently established has highly experienced personnel with many years of experience and numerous publications in the field. Physicians from the Archbishop Makarios II Hospital, the Larnaca General Hospital and the Limassol General Hospital are actively implicated in the project through the enrolment of volunteers and the evaluation of their clinical data. We are also very grateful to the thalassemia patient community for their willingness to participate in this project, a factor which is crucial for the success of this project.
This project is funded by the national Research Promotion Foundation/ Research and Innovation Foundation under the RESTART Programs 2016 – 2020/ Excellence Hubs (EXCELLENCE/0918/118). Also, the current study was reviewed and successfully approved by the Cyprus National Bioethics Committee (CNBC) (EEBKΕΠ/2019/87).
Molecular Genetics Thalassaemia department
Consortium:
- Host Organisation -The Cyprus Institute of Neurology and Genetics (CING)
Molecular Genetics Thalassaemia Department: Dr. Marina Kleanthous, Dr. Petros Kountouris, Dr. Panayiota Papasavva, Dr. Marios Phylactides, Dr. Savanna AndreouBioinformatics Group: Dr. George Spyrou, Dr. Anastasis Oulas
- Local Partners
The Cypriot Ministry of Health: Dr. Soteroula Christou, Dr. Maria Sitarou, Dr. Iren Savvides, Dr. Michael Hadjigavriel, Dr. Eleni Papanicolaou, Dr. Alexandra Mendoni, Dr. Panagiotis Boutsikos
- Foreign Research Organisations
Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF): Dr. Donato Bonifazi, Dr. Giovanni MigliaccioThe Royal London Hospital: Dr. Paul Telfer
The Royal London Hospital: Dr. Paul Telfer








0002.jpg)
